Classify each process as endothermic or exothermic. – Classify each process as endothermic or exothermic: delving into the intriguing world of energy exchange, where processes either absorb or release energy, shaping the very fabric of our universe. This exploration unveils the fundamental differences between endothermic and exothermic processes, providing a deeper understanding of the energy dynamics that govern our surroundings.
Endothermic processes, like thirsty sponges, eagerly absorb energy from their environment, while exothermic processes, in contrast, generously release energy into the world around them. Understanding these processes is crucial for unraveling the mysteries of chemical reactions, phase transitions, and physical phenomena.
Process Types

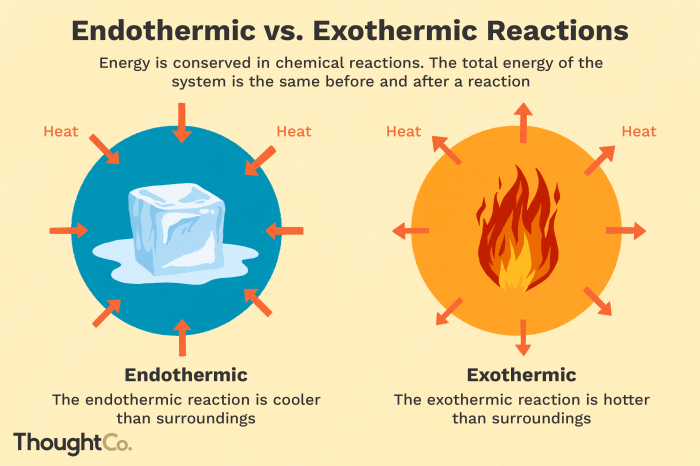
Endothermic processes are chemical or physical changes that absorb energy from their surroundings, causing a decrease in temperature. Exothermic processes, on the other hand, release energy into their surroundings, resulting in an increase in temperature.
Identifying Processes
Common endothermic processes include:
- Melting
- Vaporization
- Dissolving salts in water
Common exothermic processes include:
- Freezing
- Condensation
- Combustion
| Process | Energy Change | Example |
|---|---|---|
| Melting | Endothermic | Ice melting into water |
| Vaporization | Endothermic | Water evaporating into steam |
| Freezing | Exothermic | Water freezing into ice |
| Condensation | Exothermic | Water vapor condensing into liquid water |
Chemical Reactions, Classify each process as endothermic or exothermic.
Chemical reactions can be classified as endothermic or exothermic based on the enthalpy change (ΔH) associated with the reaction. If ΔH is positive, the reaction is endothermic, and if ΔH is negative, the reaction is exothermic.
Energy diagrams are useful for visualizing endothermic and exothermic reactions. In an endothermic reaction, the products have a higher energy level than the reactants, and energy must be absorbed from the surroundings to overcome the activation energy barrier.
Phase Transitions
Melting and vaporization are endothermic phase transitions, as they require energy to break intermolecular bonds and overcome the attractive forces holding the molecules together.
Freezing and condensation are exothermic phase transitions, as energy is released when molecules form stronger intermolecular bonds and move closer together.
- Endothermic phase transitions:Melting, vaporization
- Exothermic phase transitions:Freezing, condensation
Physical Processes
Some physical processes can also be endothermic or exothermic.
Endothermic physical processes include:
- Dissolving salts in water
Exothermic physical processes include:
- Compression of gases
| Process | Energy Change | Example |
|---|---|---|
| Dissolving salts in water | Endothermic | Salt dissolving in water, cooling the solution |
| Compression of gases | Exothermic | Compressing air in a bicycle pump, heating the pump |
Detailed FAQs: Classify Each Process As Endothermic Or Exothermic.
What is the key difference between endothermic and exothermic processes?
Endothermic processes absorb energy from the surroundings, while exothermic processes release energy into the surroundings.
Can a process be both endothermic and exothermic?
No, a process cannot be both endothermic and exothermic simultaneously.
How can we determine if a chemical reaction is endothermic or exothermic?
The enthalpy change (ΔH) of a reaction indicates whether it is endothermic (ΔH > 0) or exothermic (ΔH< 0).