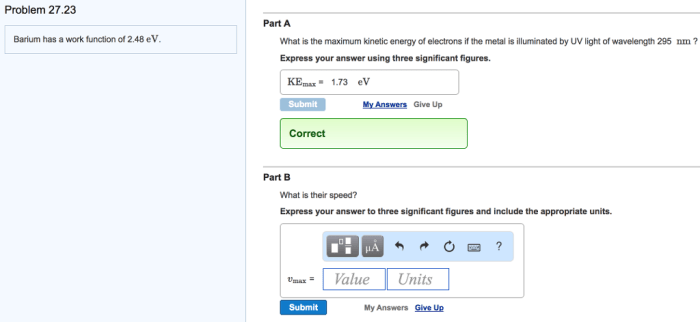
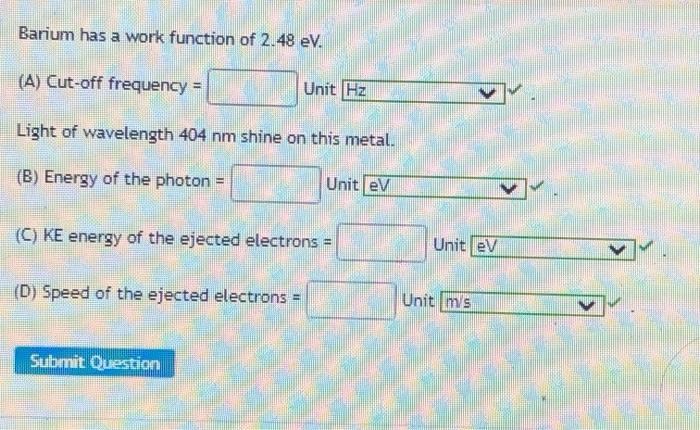
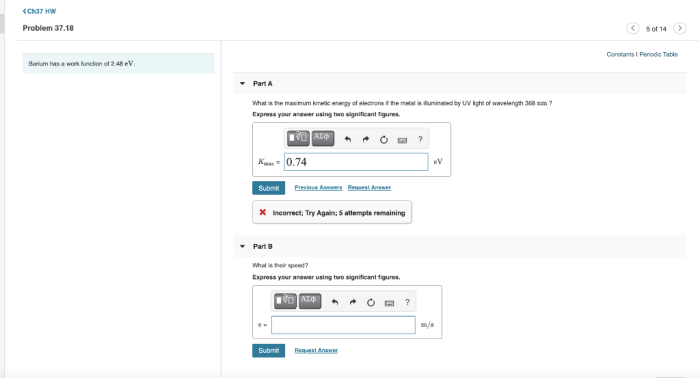
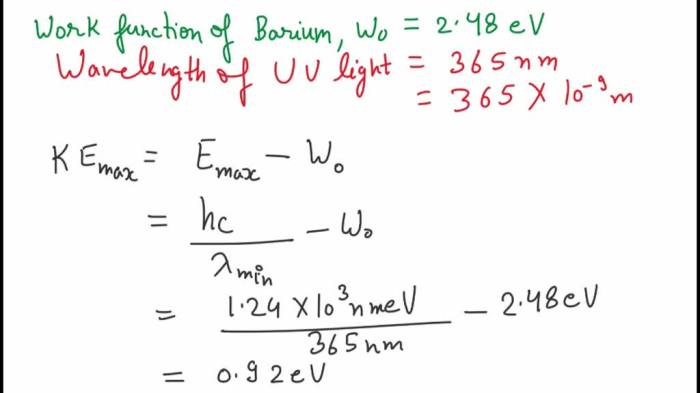
Barium has a work function of 2.48 eV, a defining characteristic that significantly influences its electronic properties and technological applications. This article delves into the significance of work function, exploring barium’s low value and its implications for various fields, particularly in thermionic emission devices.
Understanding the concept of work function provides a deeper insight into the behavior of electrons in materials, enabling the development of advanced electronic devices with tailored properties.
Work Function and its Significance
The work function is a fundamental property of materials that describes the minimum energy required to remove an electron from its surface. It plays a crucial role in understanding the behavior of electrons in materials and influences various electronic properties, such as photoemission and thermionic emission.
Barium’s Work Function
Barium has a relatively low work function of 2.48 eV, which has significant implications for its electronic properties. Compared to other common materials, barium’s work function is lower than that of most metals and semiconductors.
The low work function of barium can be attributed to its large atomic size and low ionization energy. These factors contribute to the weak binding of electrons to the barium surface.
Applications of Barium’s Low Work Function
The low work function of barium makes it an ideal material for thermionic cathodes. In thermionic emission, electrons are emitted from a heated cathode when their kinetic energy exceeds the work function of the material. Barium’s low work function allows for efficient electron emission at relatively low temperatures.
Barium-coated cathodes are widely used in vacuum tubes and electron guns, where they provide a source of electrons for various applications, including amplification, rectification, and electron microscopy.
Experimental Determination of Work Function, Barium has a work function of 2.48 ev
The work function of materials can be experimentally determined using various techniques, such as the photoelectric effect experiment. In this experiment, a light source is used to illuminate the material, and the kinetic energy of the emitted electrons is measured.
The work function can then be calculated from the energy difference between the incident light and the kinetic energy of the electrons.
For barium, the work function can be determined by measuring the threshold frequency of light that causes photoemission. The work function is equal to the energy of a photon with this threshold frequency.
Theoretical Calculations of Work Function
Theoretical models can also be used to calculate the work function of materials. These models typically involve solving the Schrödinger equation for the electrons in the material and calculating the energy difference between the highest occupied energy level and the vacuum level.
For barium, theoretical calculations based on density functional theory have yielded values for the work function that are in good agreement with experimental measurements.
Clarifying Questions: Barium Has A Work Function Of 2.48 Ev
What is the significance of work function in materials?
Work function is a crucial property that determines the energy required to remove an electron from a material’s surface. It influences various electronic phenomena, including photoemission, thermionic emission, and electrical conductivity.
Why is barium’s work function relatively low?
Barium’s low work function is attributed to its large atomic radius and low ionization energy. These factors result in a weaker binding force between barium atoms and their outermost electrons, making it easier for electrons to escape.
How is barium’s low work function utilized in practical applications?
Barium’s low work function makes it an ideal material for thermionic cathodes, which emit electrons when heated. This property is essential in vacuum tubes and electron guns, enabling the amplification and manipulation of electrical signals.