Embark on a captivating journey through the Study Guide Chapter 4 Chemistry, where you’ll unravel the mysteries of chemical reactions, solutions, and gases. This comprehensive guide will illuminate the fundamental concepts, delve into real-world applications, and equip you with practice problems to solidify your understanding.
As we delve into the realm of chemistry, we’ll explore the building blocks of matter, witness the dynamics of chemical reactions, and unravel the secrets of gases and their behavior. Prepare to be captivated as we unravel the intricacies of this fascinating field.
Introduction
This study guide for Chapter 4 of Chemistry provides a comprehensive overview of the key concepts and topics covered in the chapter. By utilizing this guide, you can effectively prepare for exams, reinforce your understanding of the material, and enhance your overall grasp of chemistry.
Chapter 4 delves into the fundamental principles of chemical reactions, exploring the various types of reactions, their mechanisms, and the factors that influence their rates. Understanding these concepts is crucial for building a solid foundation in chemistry and for further exploration in the field.
Types of Chemical Reactions
Chemical reactions are processes that involve the rearrangement of atoms and molecules, leading to the formation of new substances. This section examines the different types of chemical reactions, including synthesis, decomposition, single displacement, double displacement, and combustion reactions.
- Synthesis Reactions:Two or more substances combine to form a single product.
- Decomposition Reactions:A single substance breaks down into two or more products.
- Single Displacement Reactions:One element replaces another element in a compound.
- Double Displacement Reactions:Ions from two different compounds exchange places to form two new compounds.
- Combustion Reactions:A substance reacts with oxygen, releasing heat and light.
Reaction Mechanisms
Reaction mechanisms describe the step-by-step process by which chemical reactions occur. This section explores the different types of reaction mechanisms, including homogeneous and heterogeneous reactions, as well as the role of catalysts in influencing reaction rates.
- Homogeneous Reactions:Reactions that occur in a single phase, typically in a liquid or gas.
- Heterogeneous Reactions:Reactions that occur at the interface between two phases, such as a solid and a liquid.
- Catalysts:Substances that increase the rate of a reaction without being consumed.
Factors Affecting Reaction Rates
The rate of a chemical reaction is influenced by several factors, including temperature, concentration, surface area, and the presence of a catalyst. This section examines how these factors affect the rate of reaction and provides practical examples to illustrate their impact.
- Temperature:Increasing temperature generally increases the rate of reaction.
- Concentration:Increasing the concentration of reactants increases the rate of reaction.
- Surface Area:Increasing the surface area of reactants increases the rate of reaction.
- Catalysts:Catalysts increase the rate of reaction by providing an alternative pathway with a lower activation energy.
Key Concepts
Chapter 4 delves into the fundamental principles that govern chemical reactions, providing a solid foundation for understanding the complexities of chemistry. These concepts serve as building blocks for further exploration in the field, shaping our comprehension of chemical processes and their applications in various domains.
The chapter introduces the concept of stoichiometry, which enables us to determine the quantitative relationships between reactants and products in chemical reactions. It also sheds light on thermochemistry, exploring the energy changes that accompany chemical transformations. Additionally, the chapter delves into the concept of chemical equilibrium, explaining how reactions proceed towards a state of balance and the factors that influence this equilibrium.
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It helps us predict the amounts of reactants and products involved in a particular reaction, ensuring efficient utilization of resources and accurate experimentation.
Stoichiometric calculations are based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. By balancing chemical equations, we can determine the mole ratios between reactants and products, allowing us to calculate the exact quantities required for a reaction to proceed as desired.
Thermochemistry, Study guide chapter 4 chemistry
Thermochemistry is the study of energy changes that accompany chemical reactions. It provides insights into the energetics of chemical processes, enabling us to predict the spontaneity and feasibility of reactions.
Thermochemical equations incorporate enthalpy changes (ΔH) to quantify the heat released or absorbed during a reaction. Positive ΔH values indicate endothermic reactions that require energy input, while negative ΔH values represent exothermic reactions that release energy. Understanding thermochemistry is crucial for designing energy-efficient processes and optimizing chemical reactions.
Chemical Equilibrium
Chemical equilibrium is a dynamic state in which the forward and reverse reactions of a reversible reaction occur at equal rates, resulting in no net change in the concentrations of reactants and products. This concept is essential for understanding many chemical processes, including industrial reactions, environmental chemistry, and biological systems.
Equilibrium constants (Keq) are used to quantify the extent to which a reaction proceeds towards equilibrium. Factors such as temperature, pressure, and the presence of catalysts can influence the equilibrium position, allowing us to manipulate reactions to achieve desired outcomes.
Chemical Reactions
Chemical reactions are processes that involve the transformation of one set of chemical substances to another. These reactions are governed by specific principles, including stoichiometry and reaction rates, and have numerous applications across various fields.
There are several types of chemical reactions, each characterized by unique characteristics and mechanisms. Some common types include:
Combination Reactions
- Involve the combination of two or more substances to form a single product.
- Example: 2H 2+ O 2→ 2H 2O
Decomposition Reactions
- Involve the breakdown of a single substance into two or more products.
- Example: 2H 2O → 2H 2+ O 2
Single-Displacement Reactions
- Involve the replacement of one element in a compound by another element.
- Example: Fe + 2HCl → FeCl 2+ H 2
Double-Displacement Reactions
- Involve the exchange of ions between two compounds, resulting in the formation of two new compounds.
- Example: NaCl + AgNO 3→ NaNO 3+ AgCl
Combustion Reactions
- Involve the reaction of a substance with oxygen, typically releasing heat and light.
- Example: CH 4+ 2O 2→ CO 2+ 2H 2O
4. Solutions
Solutions are homogeneous mixtures of two or more substances. They are formed when one substance (the solute) is dissolved in another substance (the solvent). The solvent is usually a liquid, but it can also be a gas or a solid.
The solute can be a solid, liquid, or gas.
Solutions have many important properties. They are typically transparent, and they have the same composition throughout. The concentration of a solution is a measure of the amount of solute that is dissolved in a given amount of solvent. The concentration can be expressed in units of molarity, molality, or percent by mass.
Types of Solutions
There are two main types of solutions: homogeneous and heterogeneous.
- Homogeneous solutionsare solutions in which the solute is evenly distributed throughout the solvent. This means that the solution has the same composition at all points.
- Heterogeneous solutionsare solutions in which the solute is not evenly distributed throughout the solvent. This means that the solution has different compositions at different points.
Factors Affecting Solubility
The solubility of a substance in a solvent is affected by several factors, including:
- Temperature: The solubility of most substances increases with increasing temperature.
- Pressure: The solubility of gases in liquids increases with increasing pressure.
- Nature of the solute and solvent: The solubility of a substance in a solvent depends on the chemical nature of both substances.
5. Acids and Bases
Acids and bases are fundamental chemical substances that play a crucial role in various chemical reactions. They are classified based on their properties and behavior in different theories.
Arrhenius Theory
According to the Arrhenius theory, acids are substances that produce hydrogen ions (H+) when dissolved in water, while bases produce hydroxide ions (OH-) when dissolved in water. This theory is limited to aqueous solutions.
Bronsted-Lowry Theory
The Bronsted-Lowry theory defines acids as substances that donate protons (H+), and bases as substances that accept protons. This theory is more general and applies to both aqueous and non-aqueous solutions.
Lewis Theory
The Lewis theory defines acids as electron-pair acceptors, and bases as electron-pair donors. This theory is the most general and can be applied to a wide range of chemical reactions.
Properties and Characteristics
- Acids typically taste sour, turn litmus paper red, and react with metals to produce hydrogen gas.
- Bases taste bitter, turn litmus paper blue, and feel slippery to the touch.
- Acids and bases can neutralize each other, forming salts and water.
pH
pH is a measure of the acidity or basicity of a solution. It is defined as the negative logarithm of the hydrogen ion concentration. A pH of 7 is neutral, a pH below 7 is acidic, and a pH above 7 is basic.
pH is a crucial factor in many chemical and biological processes. For example, most enzymes have an optimal pH range within which they function effectively.
Gases
Gases are one of the four fundamental states of matter, along with solids, liquids, and plasmas. They are characterized by their ability to flow and expand to fill the volume of their container. Gases play a crucial role in various natural and industrial processes.
The behavior of gases can be explained by the kinetic molecular theory, which postulates that gas particles are in constant random motion and collide with each other and the walls of their container. This theory helps us understand the properties and behavior of gases, such as pressure, volume, temperature, and their relationships.
Kinetic Molecular Theory
The kinetic molecular theory is a model that describes the behavior of gases based on the following assumptions:
- Gas particles are in constant random motion.
- Gas particles collide with each other and the walls of their container elastically (without loss of energy).
- The average kinetic energy of gas particles is proportional to the absolute temperature.
- The volume occupied by gas particles is negligible compared to the volume of the container.
Gas Laws
The behavior of gases can be described by several laws, including Boyle’s law, Charles’s law, and the combined gas law. These laws relate the pressure, volume, and temperature of a gas under different conditions.
Boyle’s Law
Boyle’s law states that the pressure of a gas is inversely proportional to its volume at constant temperature. Mathematically, it can be expressed as:
P1V 1= P 2V 2
where P 1and V 1represent the initial pressure and volume, respectively, and P 2and V 2represent the final pressure and volume, respectively.
Charles’s Law
Charles’s law states that the volume of a gas is directly proportional to its absolute temperature at constant pressure. Mathematically, it can be expressed as:
V1/T 1= V 2/T 2
where V 1and T 1represent the initial volume and temperature, respectively, and V 2and T 2represent the final volume and temperature, respectively.
7. Practice Problems and Examples
Mastering chemistry involves practice and applying theoretical concepts to real-world scenarios. This section provides a series of practice problems and examples to reinforce your understanding of Chapter 4 concepts.
These problems cover various topics, including chemical reactions, solutions, acids and bases, and gases. We’ll guide you through the step-by-step solutions, demonstrating how to apply chemical principles to solve problems effectively.
7.1 Chemical Reactions
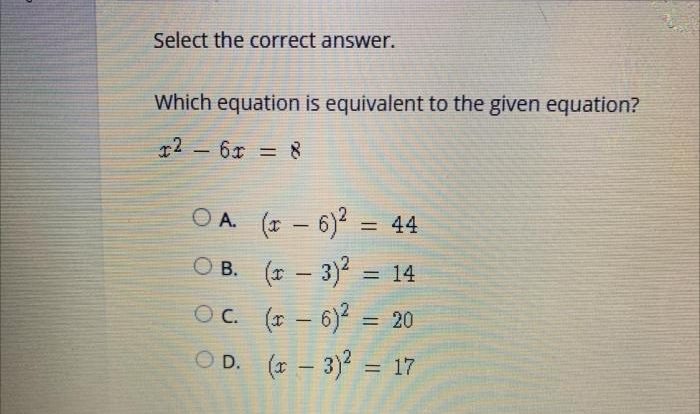
Problem 1:Balance the following chemical equation: Fe + HCl → FeCl 2+ H 2
Solution:
- Count the atoms of each element on both sides of the equation.
- Add coefficients in front of the chemical formulas to balance the atoms.
- Start with the unbalanced element and adjust coefficients until all atoms are balanced.
- The balanced equation is: Fe + 2HCl → FeCl2+ H 2
7.2 Solutions
Problem 2:Calculate the molarity of a solution prepared by dissolving 10.0 g of NaCl in 250 mL of water.
Solution:
- Convert grams of NaCl to moles: 10.0 g NaCl × (1 mol NaCl / 58.44 g NaCl) = 0.171 mol NaCl
- Convert milliliters of water to liters: 250 mL H2O × (1 L / 1000 mL) = 0.250 L H 2O
- Calculate molarity: Molarity = moles of solute / liters of solution
- Molarity = 0.171 mol NaCl / 0.250 L H 2O = 0.684 M
7.3 Acids and Bases
Problem 3:Determine the pH of a 0.10 M solution of HCl.
Solution:
- HCl is a strong acid that completely dissociates in water, releasing H+ions.
- The concentration of H +ions is equal to the concentration of HCl: [H +] = 0.10 M
- Calculate pH using the formula: pH = -log[H +]
- pH = -log(0.10) = 1.00
7.4 Gases
Problem 4:Calculate the volume of 2.00 moles of oxygen gas at a temperature of 298 K and a pressure of 1.00 atm.
Solution:
- Use the ideal gas law: PV = nRT
- Rearrange the equation to solve for volume: V = nRT / P
- Substitute the given values: V = (2.00 mol) × (0.0821 L⋅atm/(mol⋅K)) × (298 K) / (1.00 atm)
- Volume = 49.9 L
Conclusion: Study Guide Chapter 4 Chemistry
Chapter 4 of Chemistry has provided a comprehensive overview of key concepts that are essential for understanding the field. These concepts form the foundation for further exploration in chemistry and its applications in various real-world scenarios.
By mastering the concepts discussed in this chapter, you will gain a deeper understanding of the behavior of matter, chemical reactions, solutions, acids and bases, and gases. This knowledge will equip you to tackle more advanced topics in chemistry and prepare you for further studies or careers in the field.
Further Study Resources
To enhance your understanding and delve deeper into the concepts covered in Chapter 4, consider exploring the following resources:
- Textbooks and online learning platforms
- Scientific journals and research papers
- Chemistry forums and discussion groups
li>Interactive simulations and experiments
Applications in Real-World Scenarios
The concepts discussed in Chapter 4 have numerous applications in real-world scenarios, including:
- Understanding the behavior of substances in everyday life (e.g., cooking, cleaning, medicine)
- Developing new materials and technologies
- Solving environmental problems
- Advancing fields such as medicine, engineering, and agriculture
Frequently Asked Questions
What is the purpose of a study guide for Chapter 4 of Chemistry?
The study guide for Chapter 4 of Chemistry serves as a valuable tool to reinforce your understanding of the chapter’s key concepts, providing a structured approach to learning and practice.
What are the key topics covered in Chapter 4 of Chemistry?
Chapter 4 of Chemistry encompasses a wide range of topics, including chemical reactions, solutions, acids and bases, and gases. You’ll explore the principles governing these concepts and their practical applications.
How can I use the practice problems in the study guide?
The practice problems provided in the study guide are designed to test your comprehension of the chapter’s concepts. By working through these problems, you can identify areas where you need further review and strengthen your problem-solving skills.